

3TR Precis-The-RA is a prospective open label randomised controlled trial, involving patients that have not responded to cDMARDs.
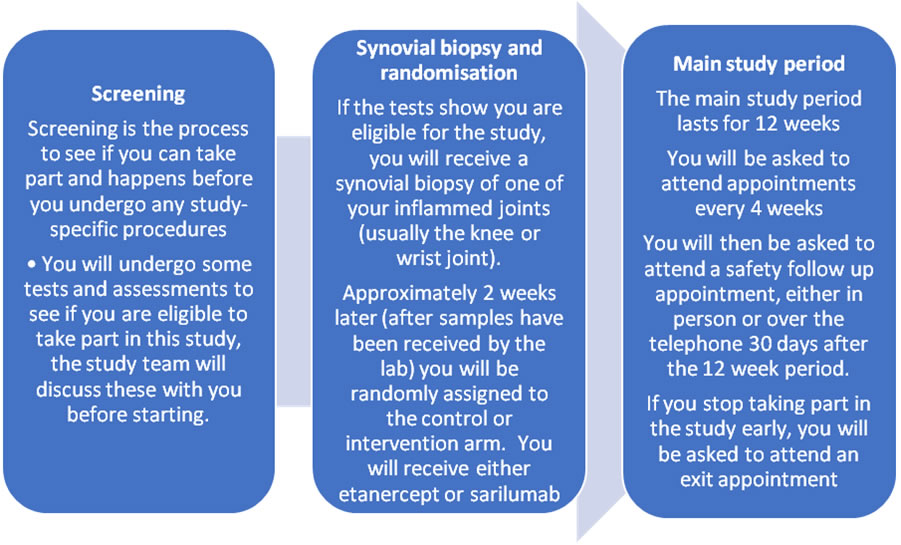
The study involves 7 visits and is carried out over 4 months usually in line with standard routine clinical visits. The study has been broken down in the following diagram to 3 parts:
Disclaimer: always refer to the latest version in the protocol

This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking(JU) under grant agreement No 831434.
The JU receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA.
The content of this website reflects only the author's view and JU is not responsible for any use that may be made of the information it contains.